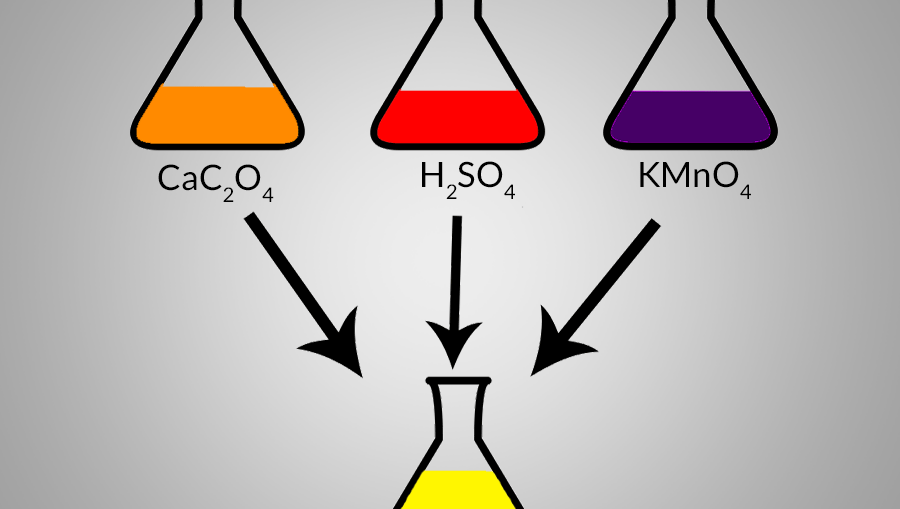
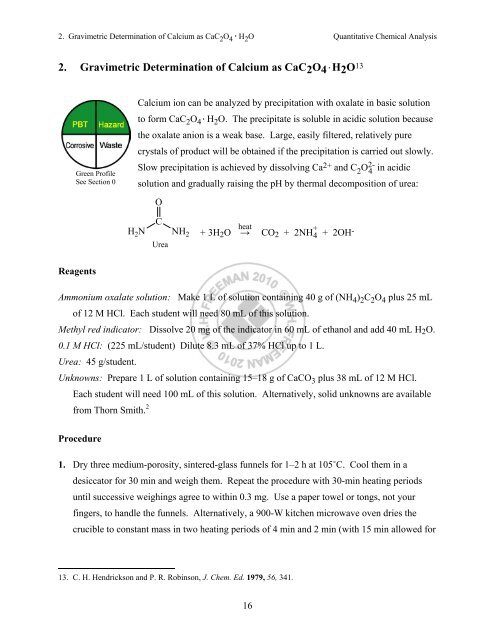
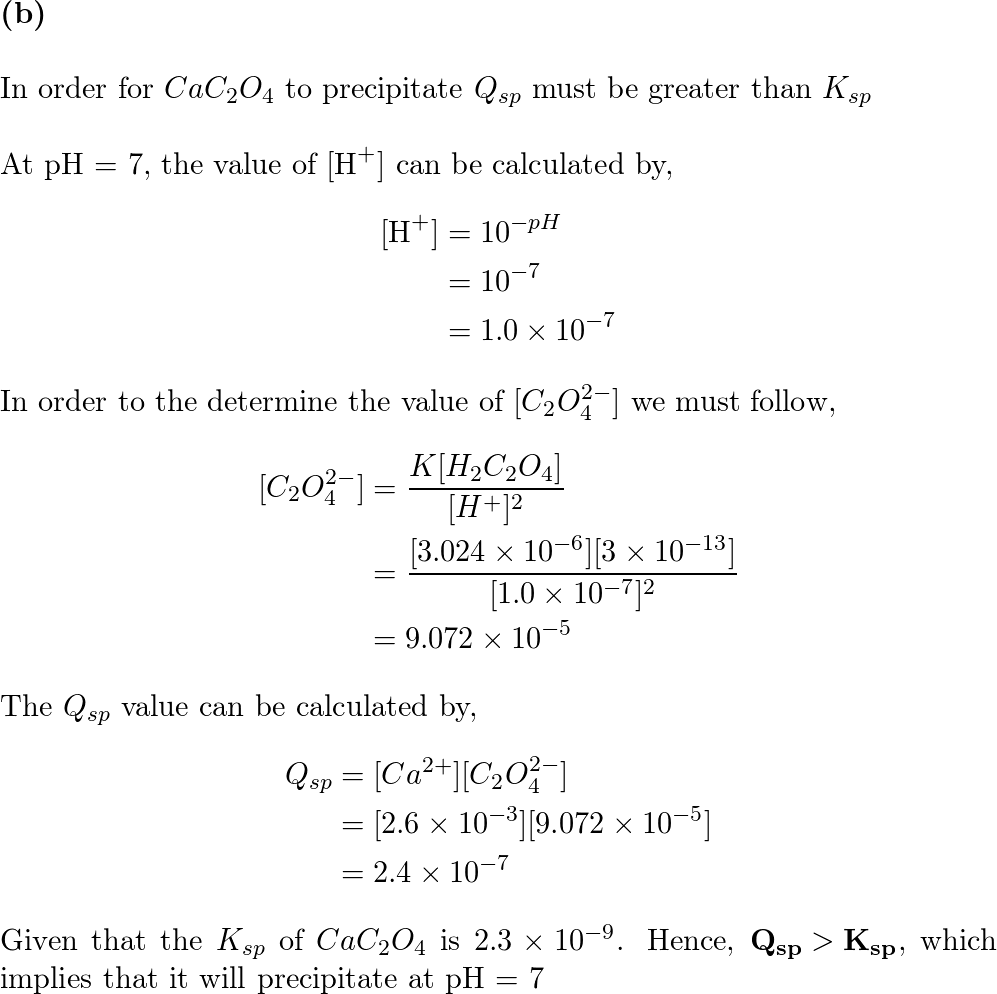SOLVED: Consider a saturated solution of calcium oxalate, CaC2O4, in which the following equilibrium can occur: CaC2O4(s) ⇌ Ca2+ (aq) + C2O42- (aq) Ksp = 1.3 × 10-8 C2O42- (aq) + H2O (

A 0.60 g sample consisting of only CaC2O4 and MgC2O4 is heated at 500 ^∘ C , converting the two salts of CaCO3 and MgCO3 . The sample then, weighs 0.465 g .

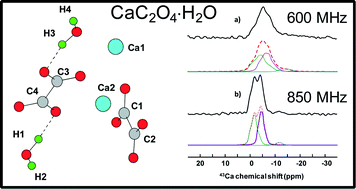
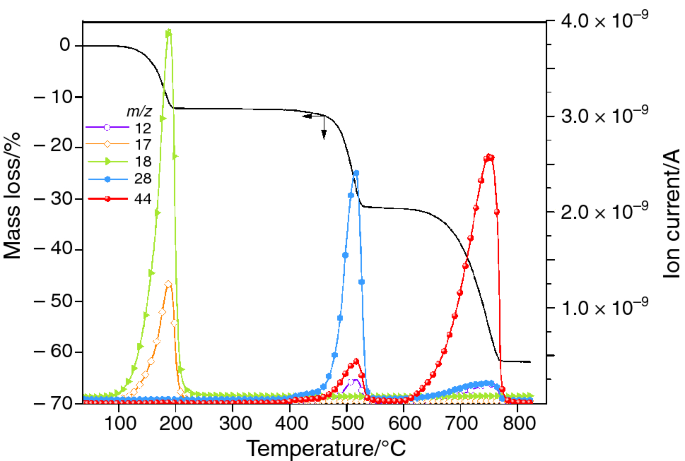
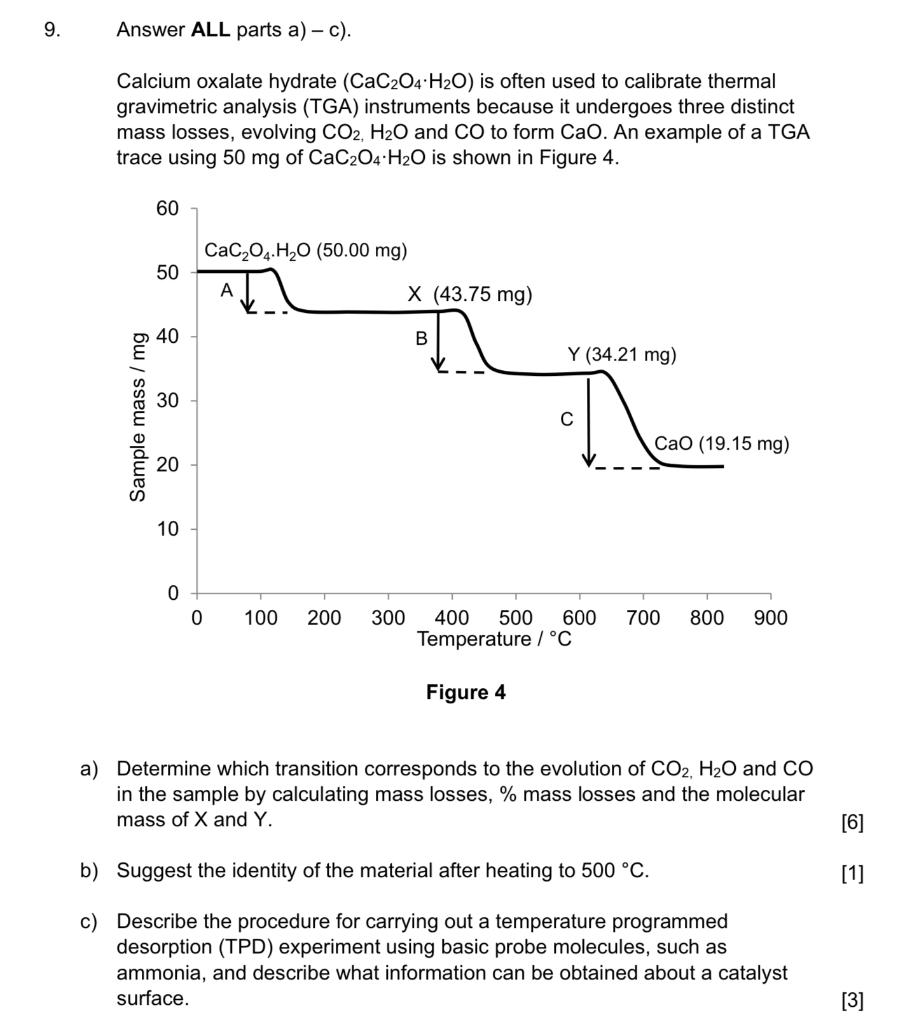
Thermal Decomposition of CaC2O4·H2O Studied by Thermo-Raman Spectroscopy with TGA/DTA | Analytical Chemistry
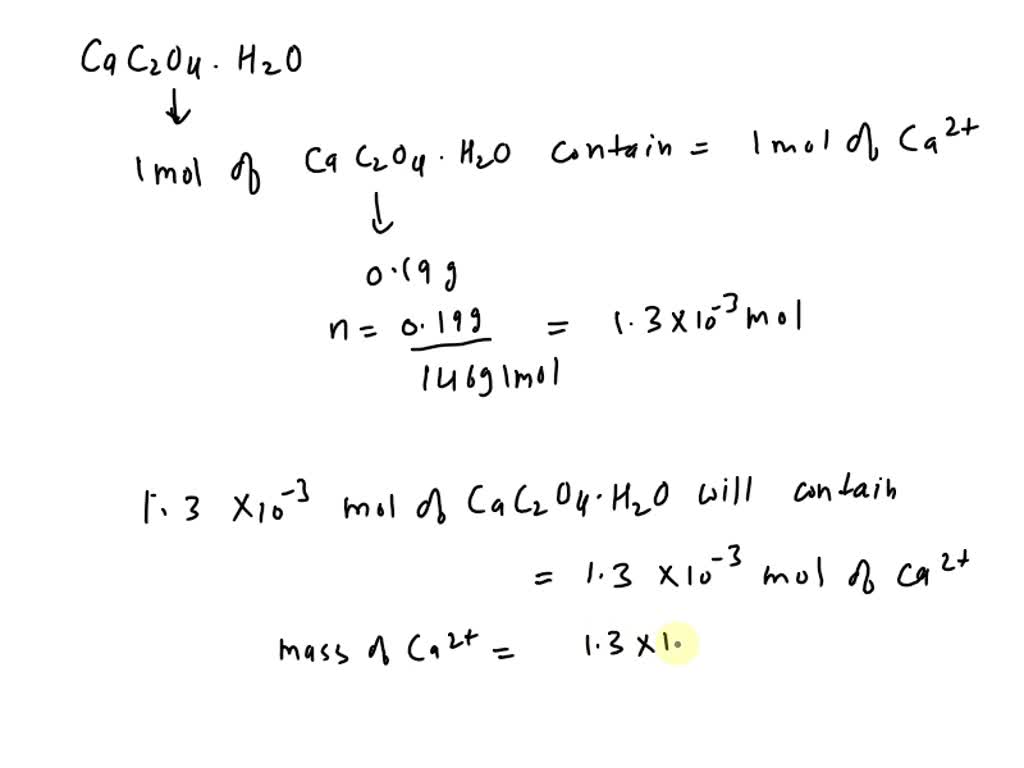
SOLVED: What is the concentration of calcium (Ca2+) in calcium oxalate monohydrate (CaC2O4.H2O). Ca2+(aq) + C2O42-(aq) + H2O (l) → CaC2O4·H2O (s) The mass of calcium oxalate monohydrate is 0.190grams. what is

A 0.60 g sample consisting of only CaC2O4 and MgC2O4 is heated at 500 ^∘ C , converting the two salts of CaCO3 and MgCO3 . The sample then, weighs 0.465 g .