
Coordination numbers of the Fe 3 + and Fe 2 + ions calculated for their... | Download Scientific Diagram
PPT - Iron (Fe 2+ /Fe 3+ ) Transport and Trafficking in Mammals Bertini et al Ch. 5 and 8 PowerPoint Presentation - ID:432903

Inhibition of Fe2+- and Fe3+- induced hydroxyl radical production by the iron-chelating drug deferiprone - ScienceDirect

Homogeneous photocatalytic Fe3+/Fe2+ redox cycle for simultaneous Cr(VI) reduction and organic pollutant oxidation: Roles of hydroxyl radical and degradation intermediates - ScienceDirect

Exploring selective recognition between Fe2+, Fe3+ and their implementation in bio-imaging: A combined spectroscopic and theoretical investigation - ScienceDirect

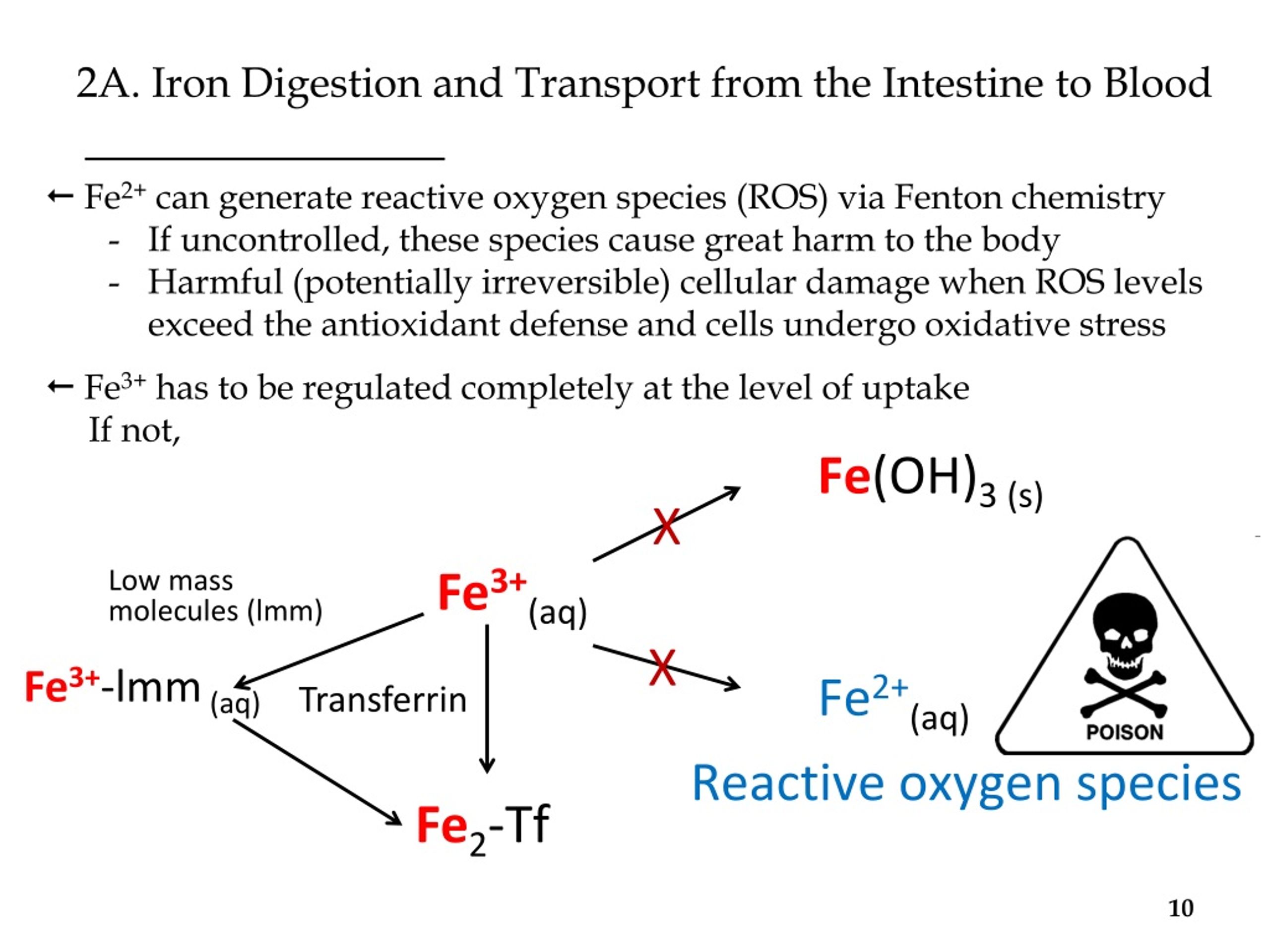
Schematic of intestinal iron uptake. Fe3+ in the intestinal lumen is... | Download Scientific Diagram

Given standard electrode potentials, Fe^2 + 2e^-→ Fe, E^∘ = - 0.440 V Fe^3 + + 3e^-→ Fe, E^∘ = - 0.036 V The standard electrode potential (E^∘) for Fe^3 + + e^-→ Fe^2 + is:

Fe2+/Fe3+ Cycling for Coupling Self‐Powered Hydrogen Evolution and Preparation of Electrode Catalysts - Chen - 2022 - Angewandte Chemie International Edition - Wiley Online Library

Double-enzymes-mediated Fe2+/Fe3+ conversion as magnetic relaxation switch for pesticide residues sensing - ScienceDirect

Comparison of Fe 3 + /Fe 2 + ratios determined for standard basaltic... | Download Scientific Diagram

SOLVED: QUESTion points Save Anawor If a Galvanic cell is created using the Ag+/Ag and Fe2+/Fe3t half reactions, what is the net reaction for this electrochemical cell? Fe3-(aq) Ag"(aq) Fe?+(aq) Agls) Fe?-(aq)




.PNG)



