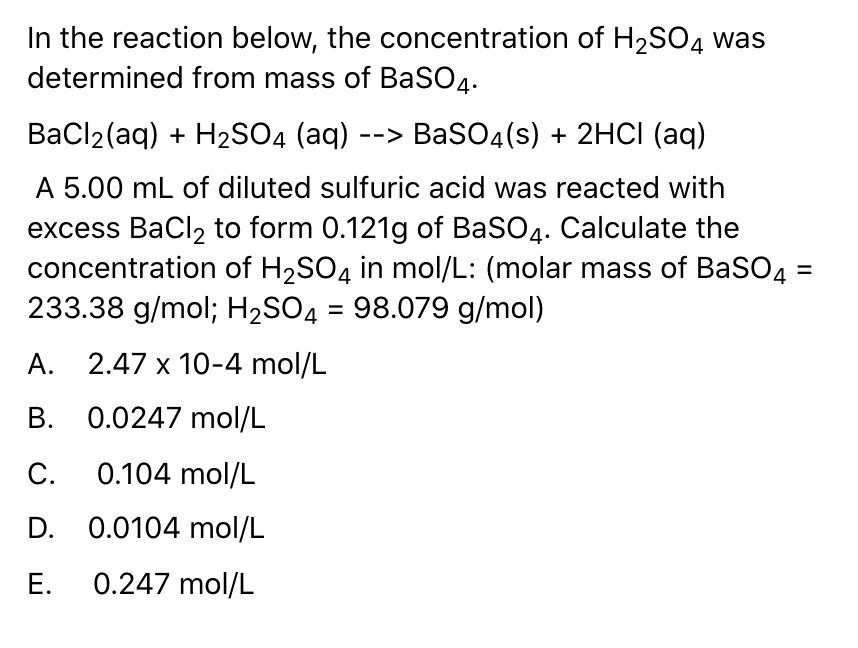
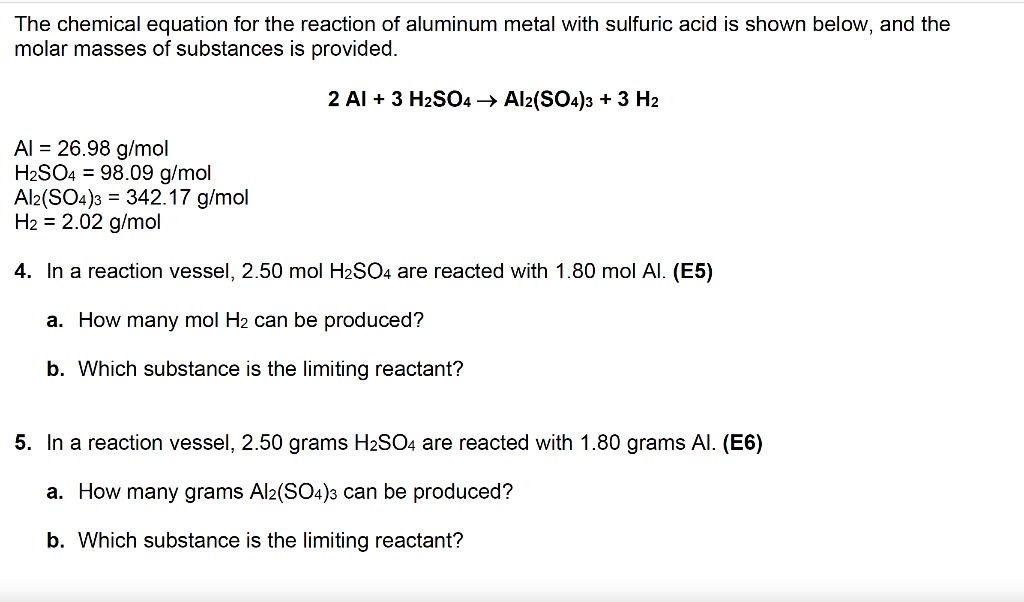
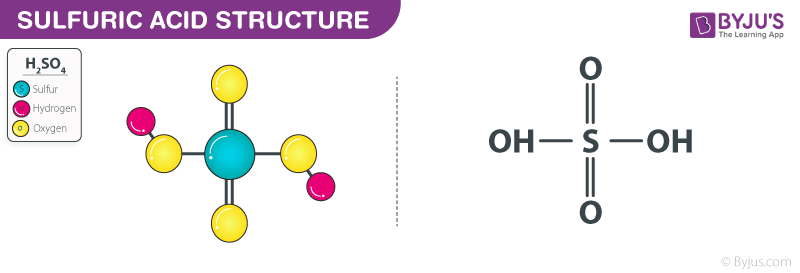
Gas permeance change of each substrate of 1 mol L −1 H2SO4 immersion... | Download Scientific Diagram

The molecular mass of H2SO4 is 98 amu. Calculate the number of moles of each elements in 294 g of H2SO4

Alumminum hydroxide reacts with sulfuric acid as follows: 2Al(OH)3+H2SO4-->Al2(SO4)+6H2O. Which reagent is the limiting reactant when 0.500 mol Al(OH)3 and 0.500 mol H2SO4 are allowed to react? How ma | Homework.Study.com
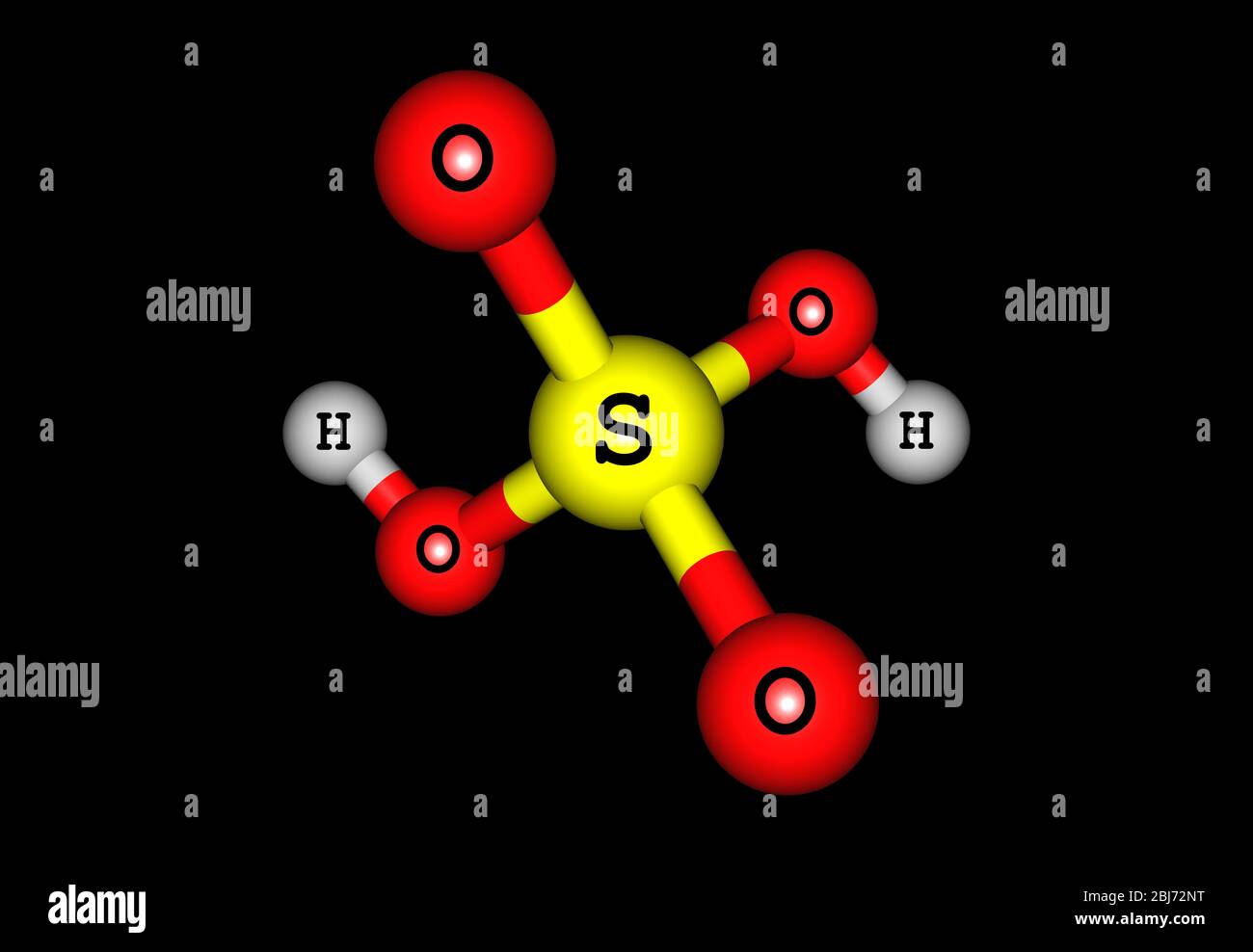
Sulfuric acid (sulphuric acid) is a highly corrosive strong mineral acid with the molecular formula H2SO4. It is a pungent-ethereal, colorless to slig Stock Photo - Alamy

Question Video: Calculating the Volume of Sulfuric Acid That Completely Neutralizes a Given Volume and Concentration of Sodium Hydroxide | Nagwa

SOLVED: 4. What is the molar mass of 3.25 mol of H2SO4 Points) Enter your answer 5. What is the molar mass of 2.5 mol of (C3H5)2 ? Points) Enter your answer
100 ml of 3 mol H2SO4 reacts with 100 ml of 3 mol NaOH . What is the enthalpy of neutralisation of reaction?