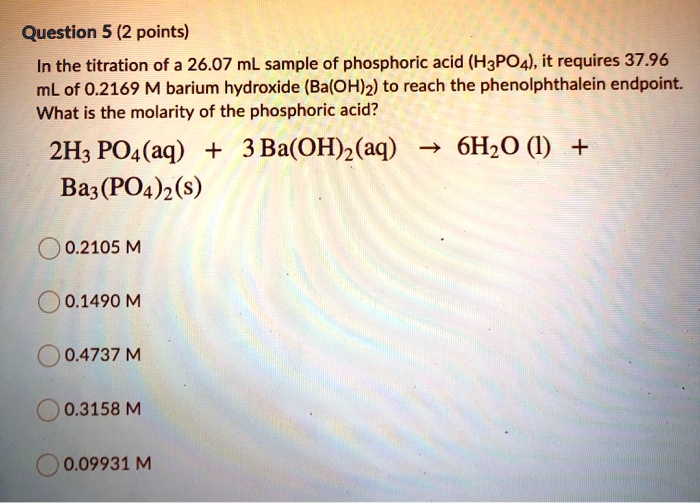
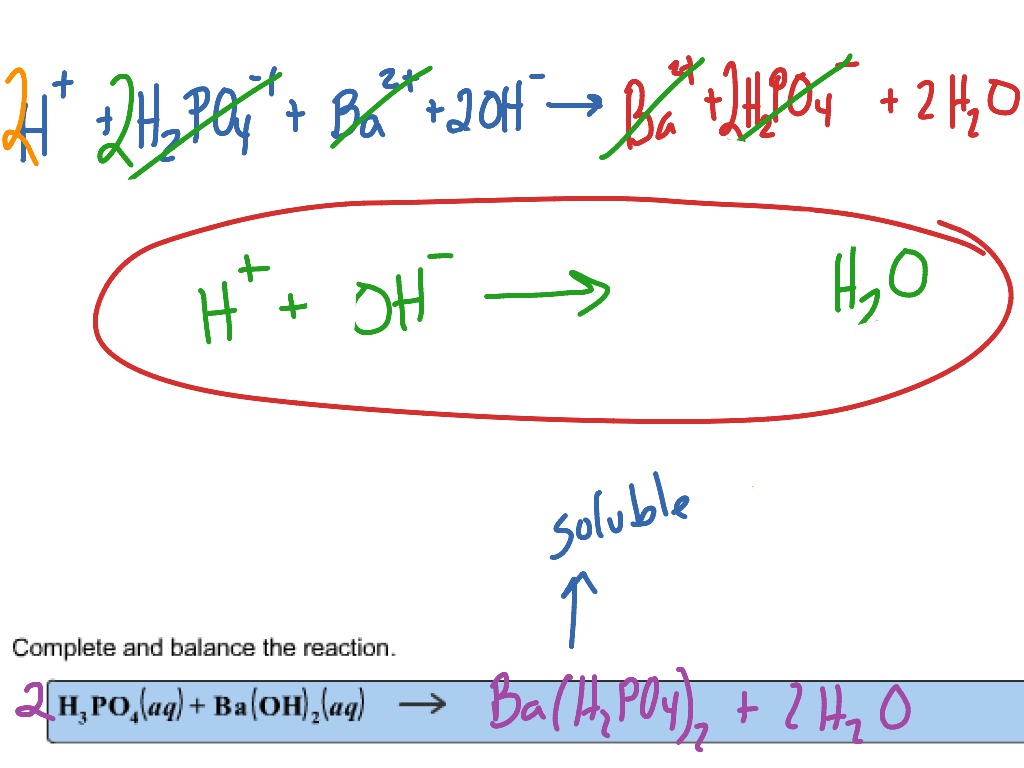
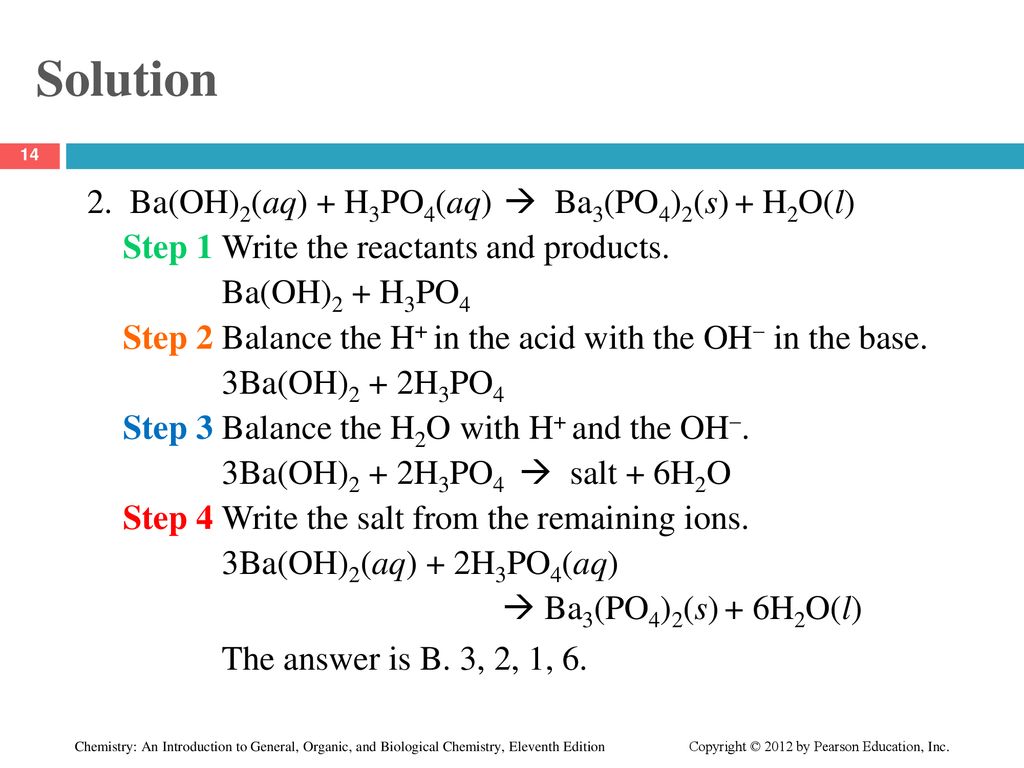
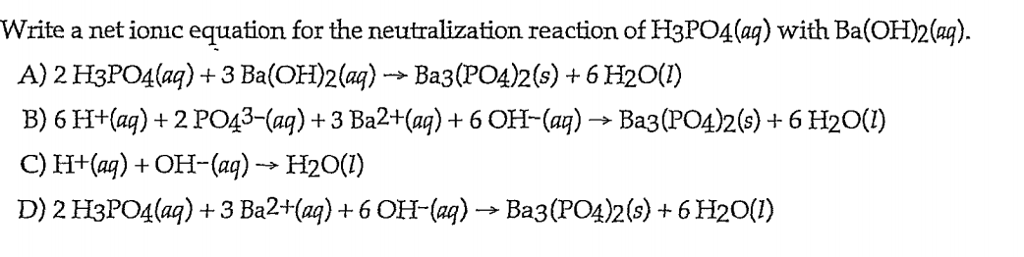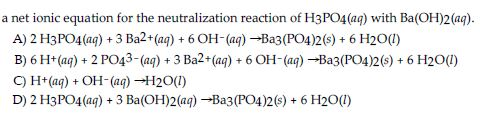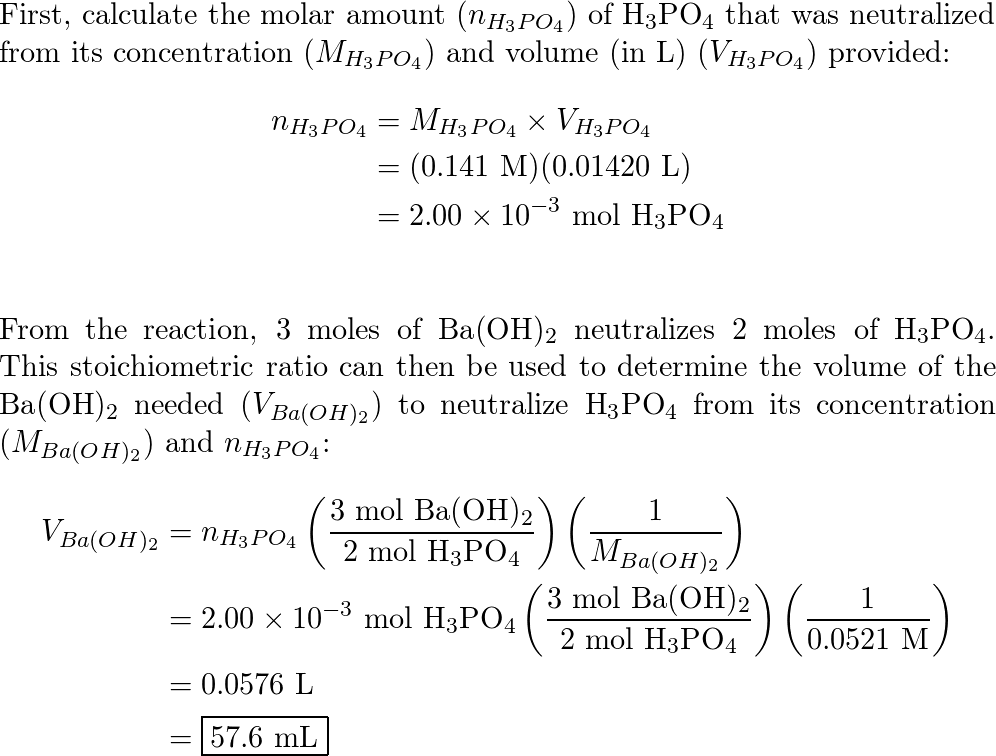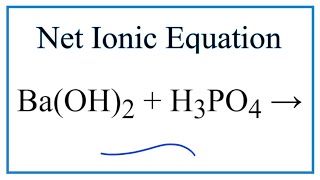✓ Solved: What volume of 0.0521 M Ba(OH) 2 is required to neutralize exactly 14.20 mL of 0.141 M H 3...

SOLVED: If 30.0 mL of 0.15 M Ba(OH)2 was needed to neutralize 50.0 mL of an H3PO4solution. What is the concentration of the original H3PO4 solution?

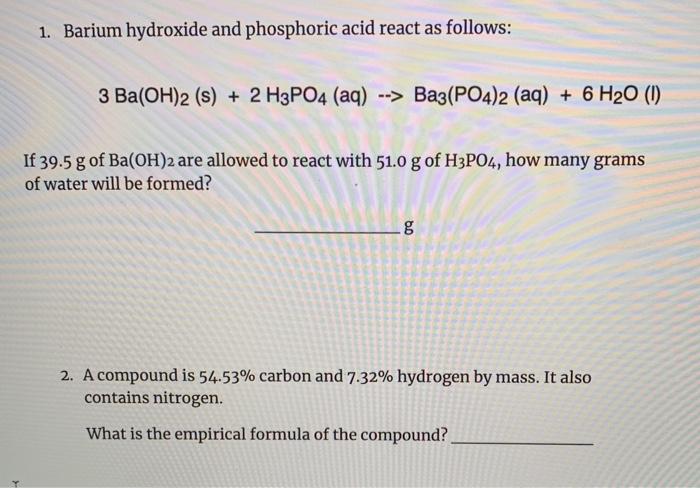
SOLVED: Barium hydroxide and phosphoric acid react as follows: 3 Ba(OH)2(s) + 2 H3PO4(aq) –> Ba3(PO4)2(aq) + 6 H2O(l) If 39.5 g of Ba(OH)2 are allowed to react with 51.0 g of
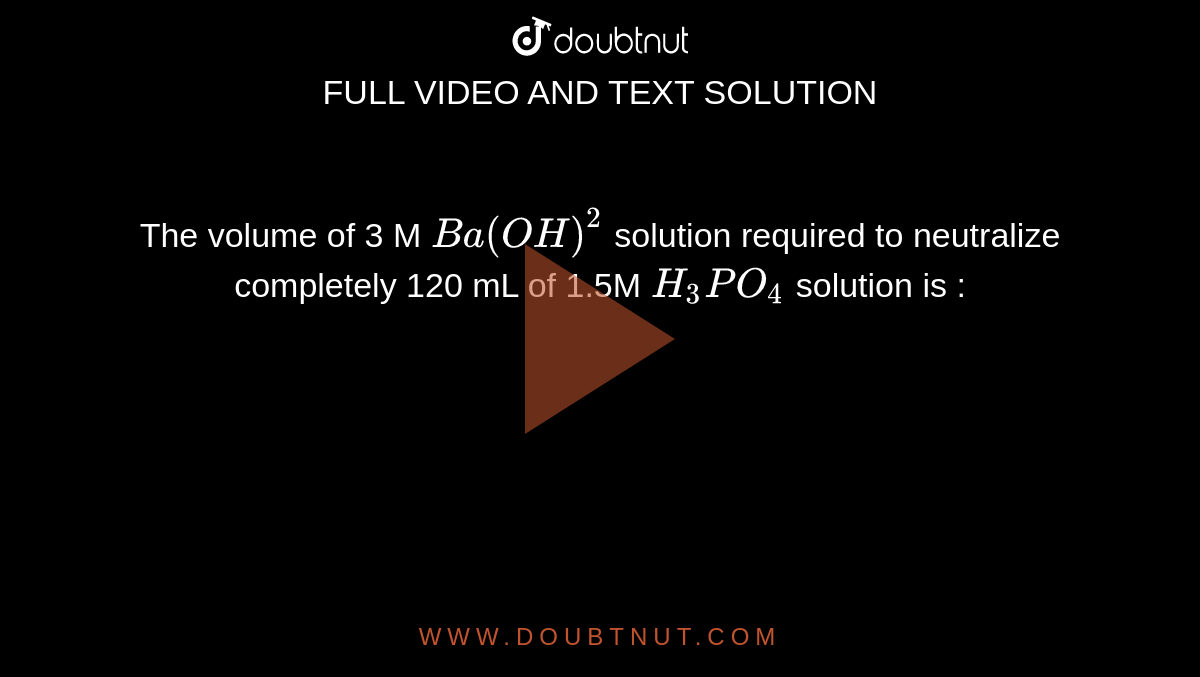
The volume of 3 M Ba(OH)^(2) solution required to neutralize completely 120 mL of 1.5M H(3)PO(4) solution is :
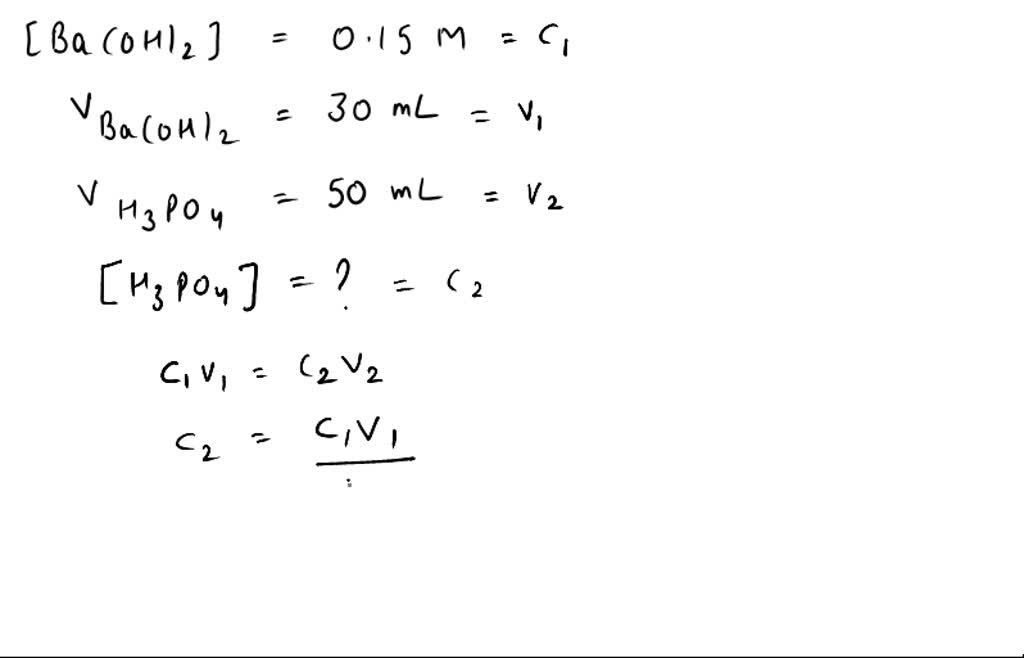
SOLVED: If 30.0 mL of 0.15 M Ba(OH)2 was needed to neutralize 50.0 mL of an H3PO4 solution. What is the concentration of the original H3PO4 solution?
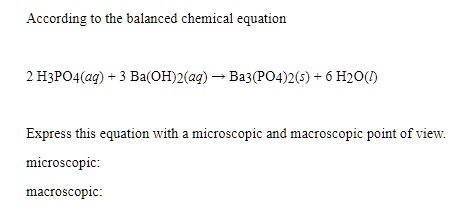
SOLVED: According to the balanced chemical equation 2 H3PO4(aq) Ba(OH) (aq) Ba3(PO4)2(5) Hzo() Express this equation With microscopic and macroscopic point of view: micfoscopic: macroscopic: