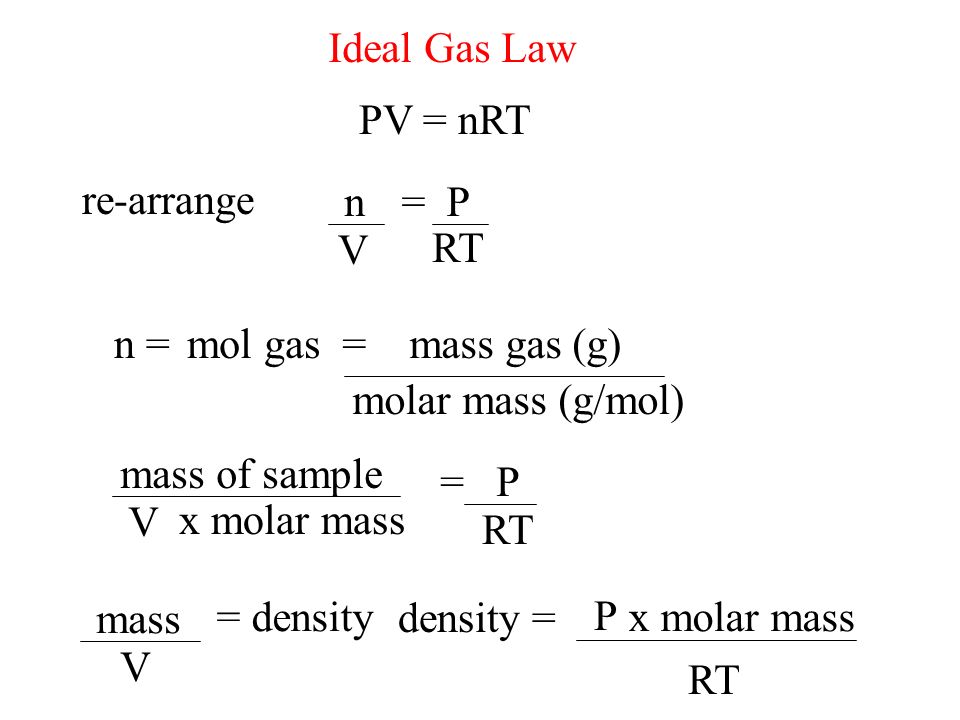
Ideal Gas Law PV = nRT re-arrange n V = P RT n = molar mass (g/mol) mol gas= mass gas (g) mass of sample V x molar mass = P RT =

N moles of an ideal diatomic gas are in a closed cylinder at temperature T. Suppose, on supplying heat to the gas, its temperature remains constant, but n moles get dissociated into

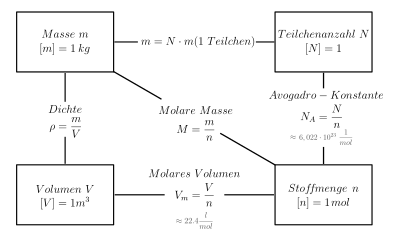
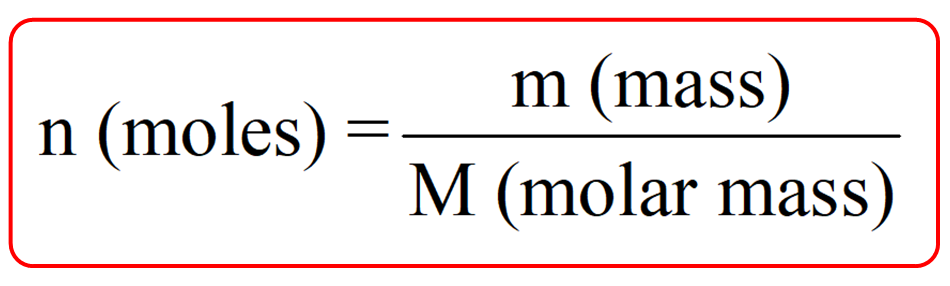
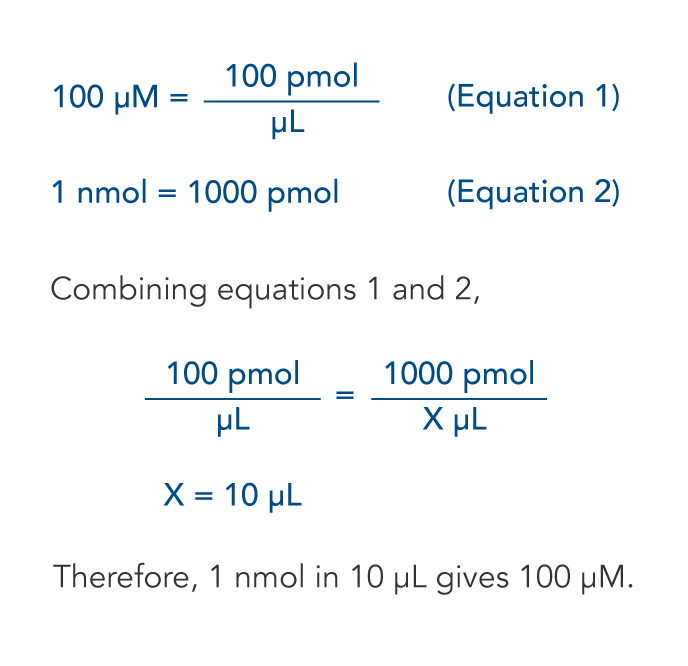
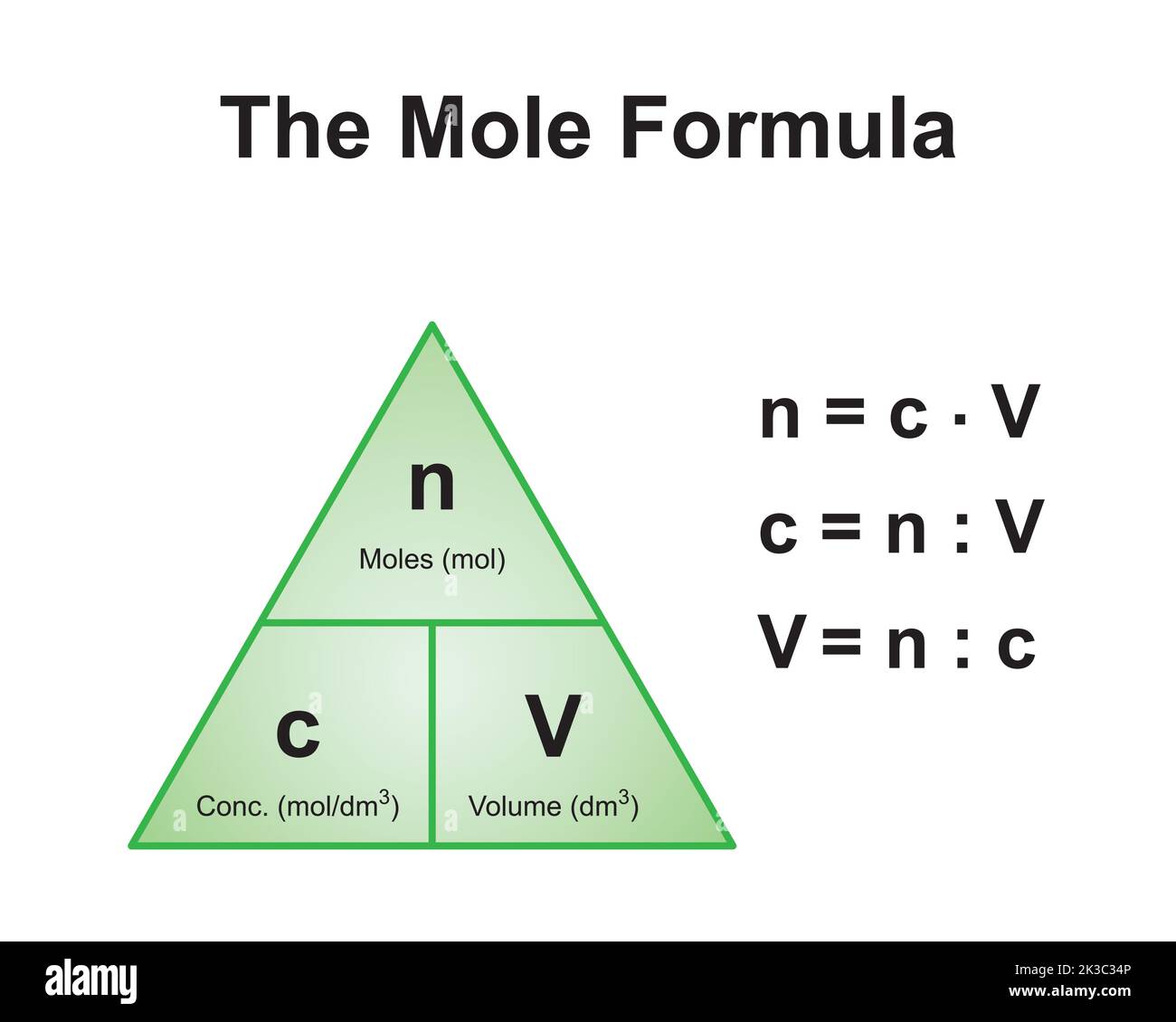

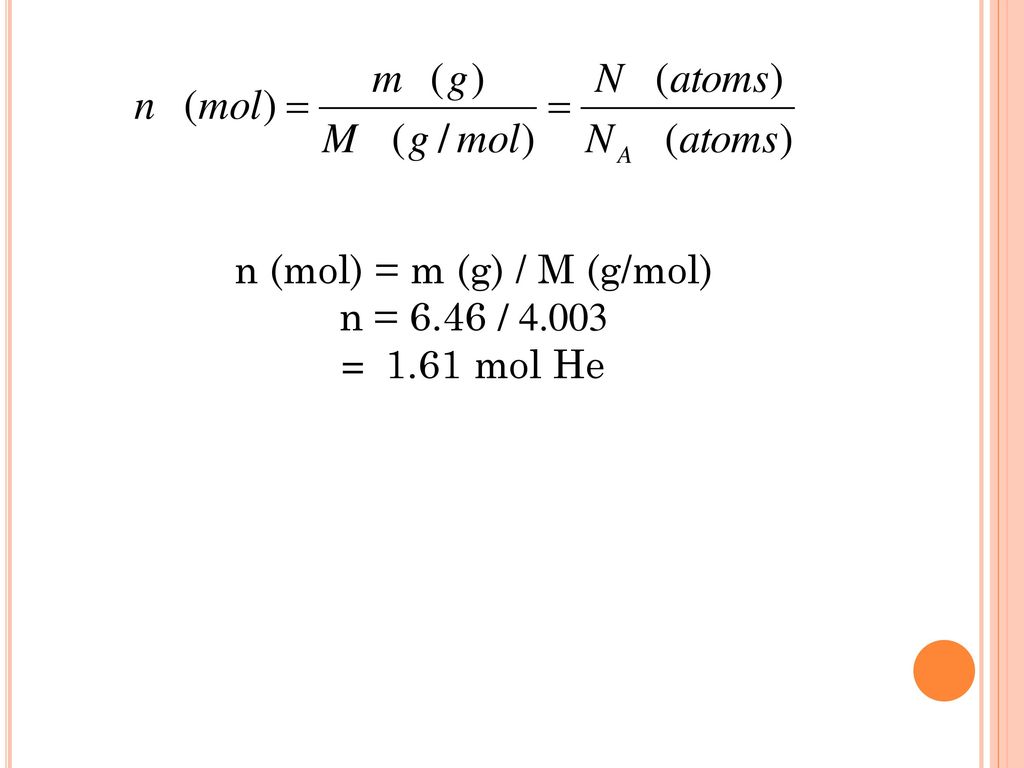


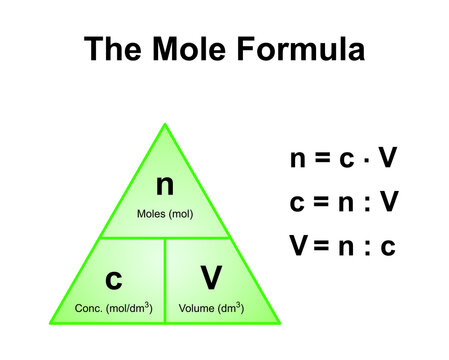
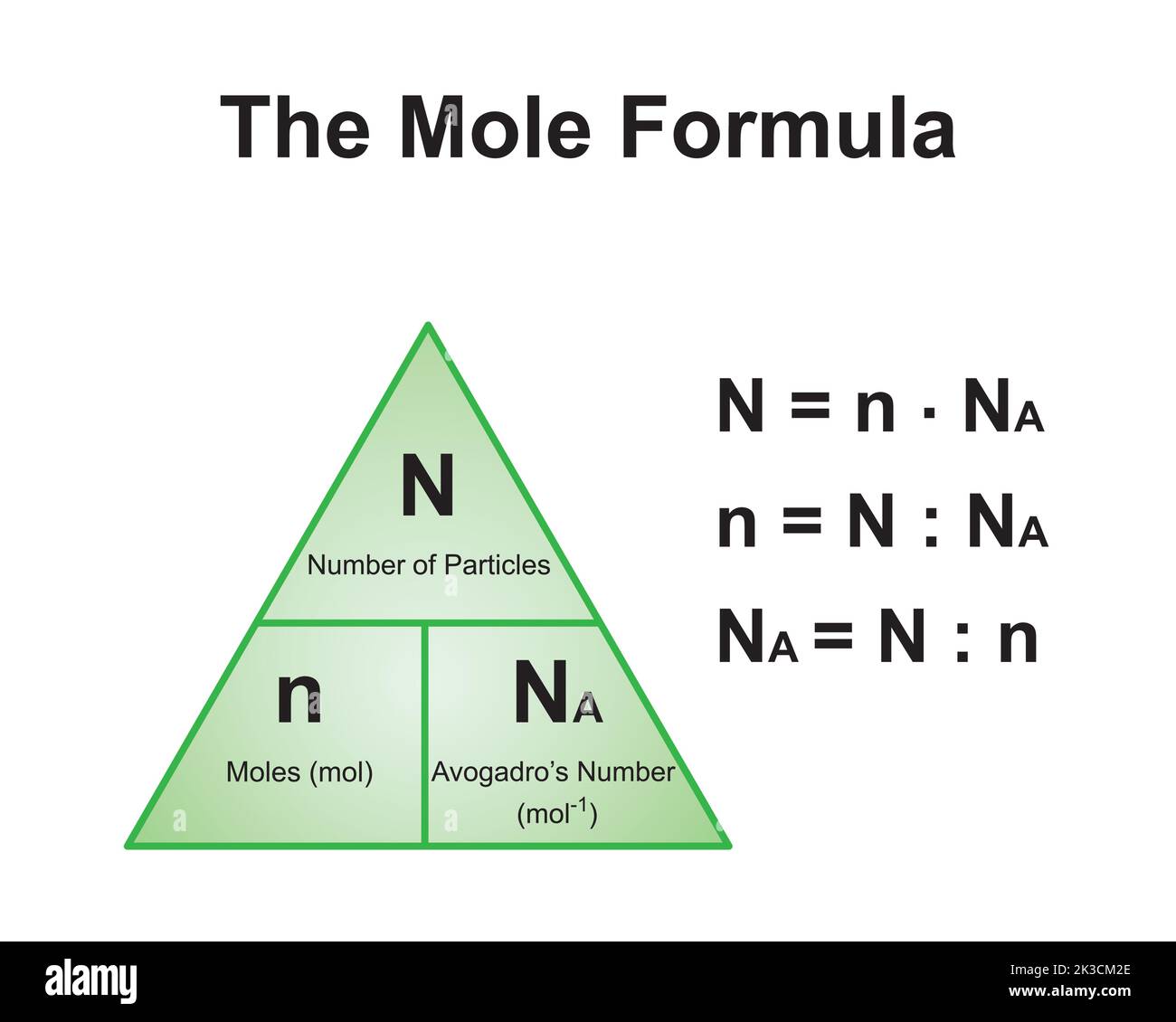


![Stoffmengekonzentration • Formel und Berechnung · [mit Video] Stoffmengekonzentration • Formel und Berechnung · [mit Video]](https://d3f6gjnauy613m.cloudfront.net/system/production/videos/000/273/7f0e9b8cbc6cc89263e18edb28ff916bb23553f0/poster_Thumbnail_Stoffmengenkonzentration.png?1669032134)