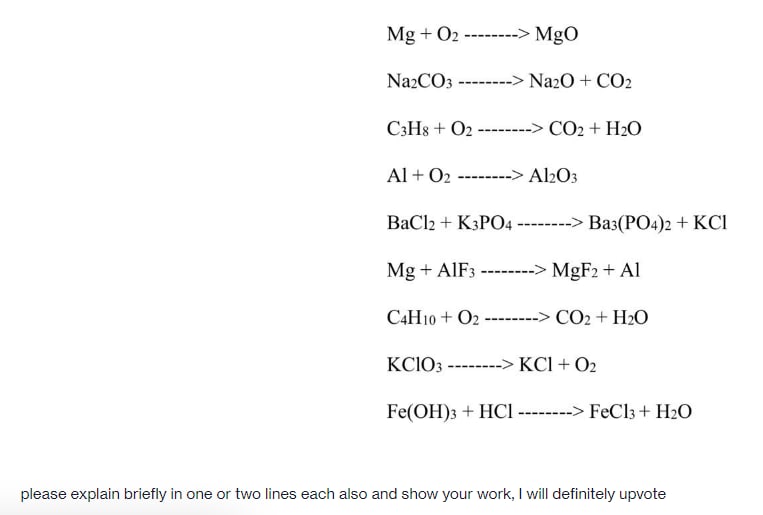
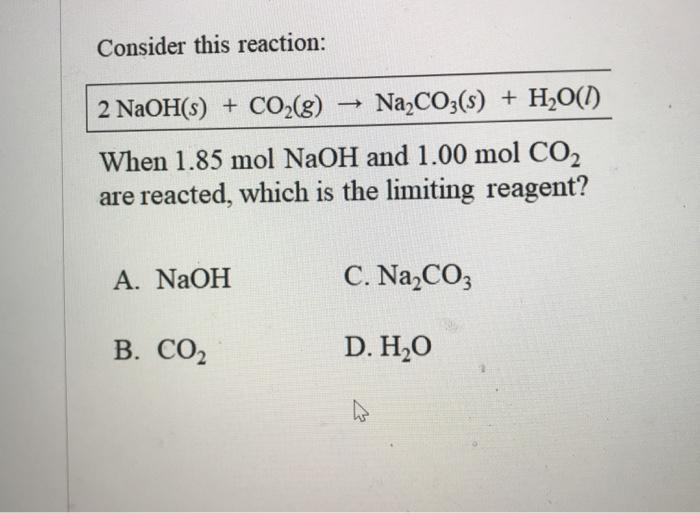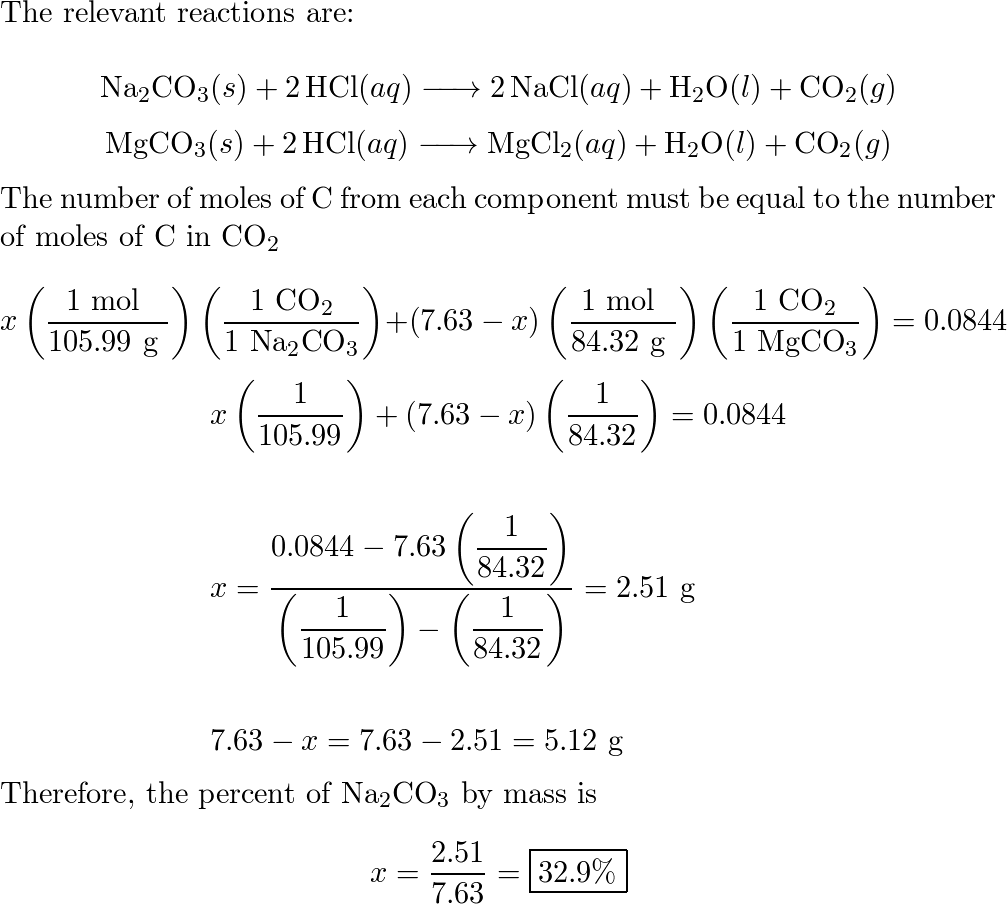40. A 2g sample containing Na2CO3 and NaHCO3 loses 0.248g when heated to 300^° c,the temperature at which NaHCO3 decomposes to Na2CO3,CO2 AND H2O.what is the precentage of Na2CO3 in the given

C-CO-CO2-Na2CO3-NaCl-AgClпомогите , объясните как делать :(завтра контроха (((( - Школьные Знания.com

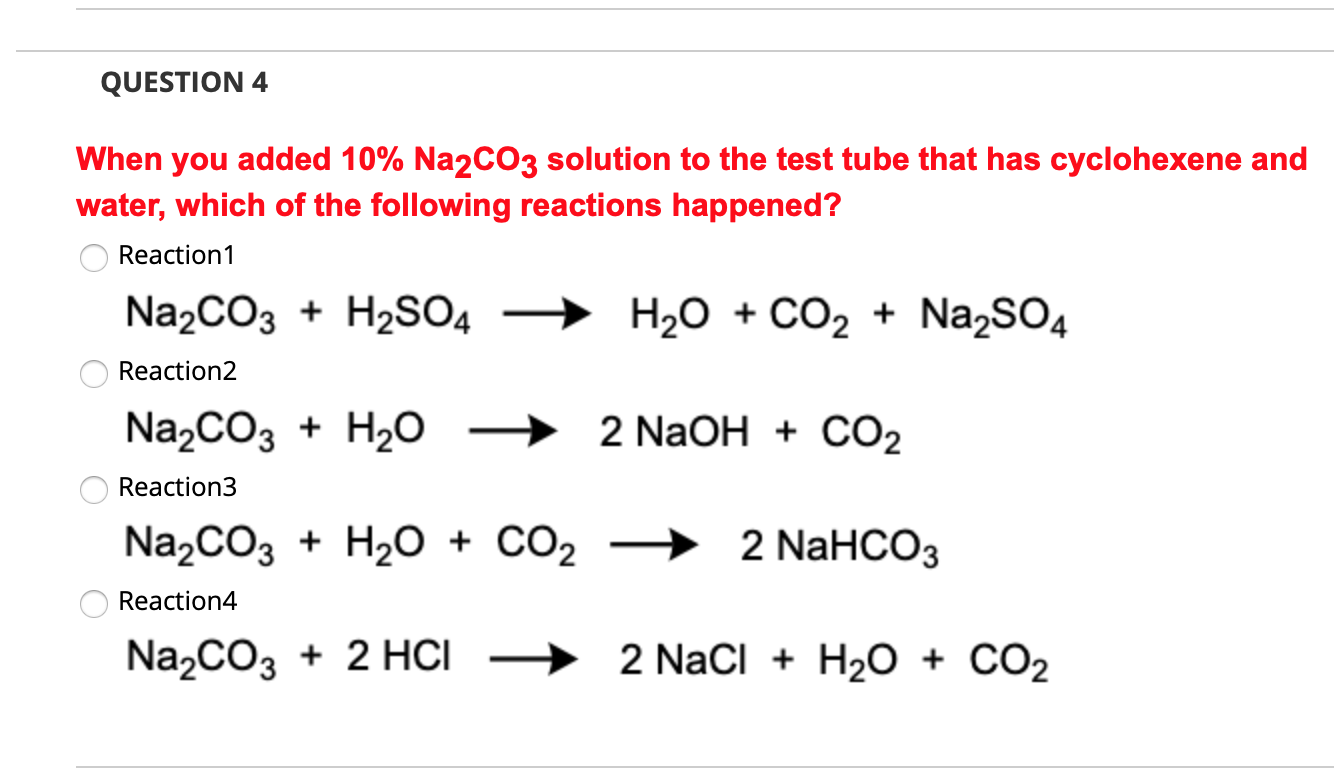
Осуществите превращения по схеме: 1) CO2 → H2CO3 → Na2CO3 → CO2 2) CaCO3 → CO2 → NaHCO3 → - Школьные Знания.com
Na2CO3 + 2HCl → 2NaCl + CO2 + H20 . The hydrochloric acid (HCl) in the gastric juice has a pH of 2.00. How many cm3 of 0.5M of sodium carbonate ( Na2CO3)

Welcome to Chem Zipper.com......: When 15 gm of NaCl and Na2CO3 is heated with dilute HCl, 2.5 gm of CO2 is evolved at NTP. Calculate percentage composition of the original mixture.

6.2g of a sample containing Na2CO3, NaHCO3 and non-volatile inert impurity on gentle heating loses 5% of its mass due to reaction 2NaHCO3 rarr Na2 CO3 + H2 O + CO2 .

⚗️This chemical equation represents a chemical reaction. Na2CO3 + 2HCl - 2NaCl + CO2 + H20 Which - Brainly.com