✓ Solved: When 4-chlorobutane-1-thiol is treated with a strong base such as sodium hydride, NaH, tetrahydrothiophene...

When the halohydrin is treated with NaH, a product of molecular formula C_4H_8O is formed. Draw the structure of the product and indicate its stereochemistry. | Homework.Study.com

The hydride ion ( H^ - ) is stronger base than OH^ - ion. Which of the following reaction will occurs if sodium hydride (NaH) is dissolved in water?

Complications from dual roles of sodium hydride as a base and as a reducing agent. | Semantic Scholar

Complications from dual roles of sodium hydride as a base and as a reducing agent. - Abstract - Europe PMC

OneClass: Predict the structures of BOTH bronsted acid base reaction of NaH with typical thiol. What ...

I'm having trouble determining what the base in this E1 reaction is , anyone has an idea ? : r/OrganicChemistry
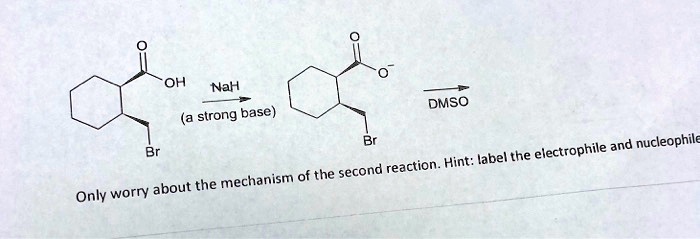
SOLVED: OH NaH DMSO strong base) and nucleophile Hint: label the electrophile second reaction mechanism of the Only worry about the

The hydride ion H^(-) is a stronger base than its hydroxide ion OH^(-). Which of the following reactions will occurs if sodium hydride (NaH) is dissolved in water ?


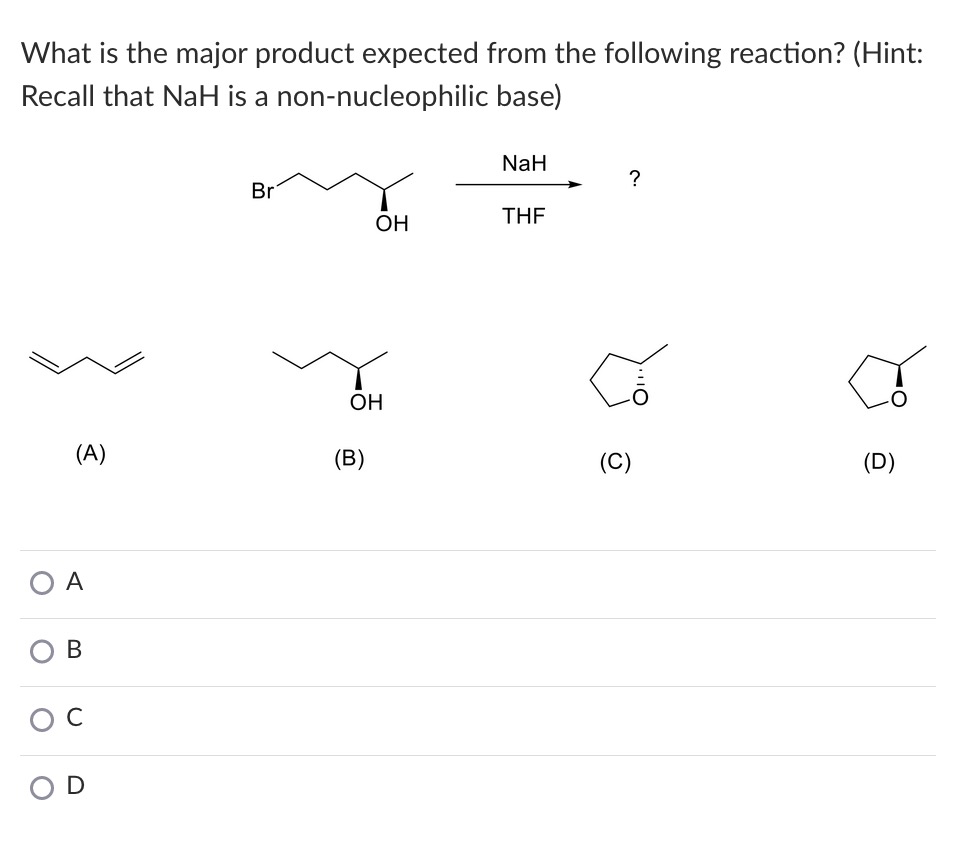
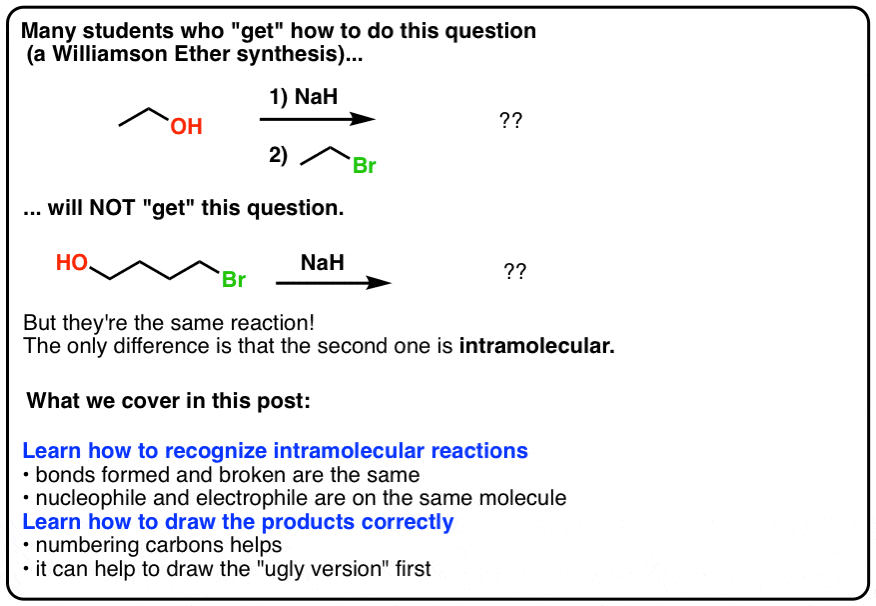

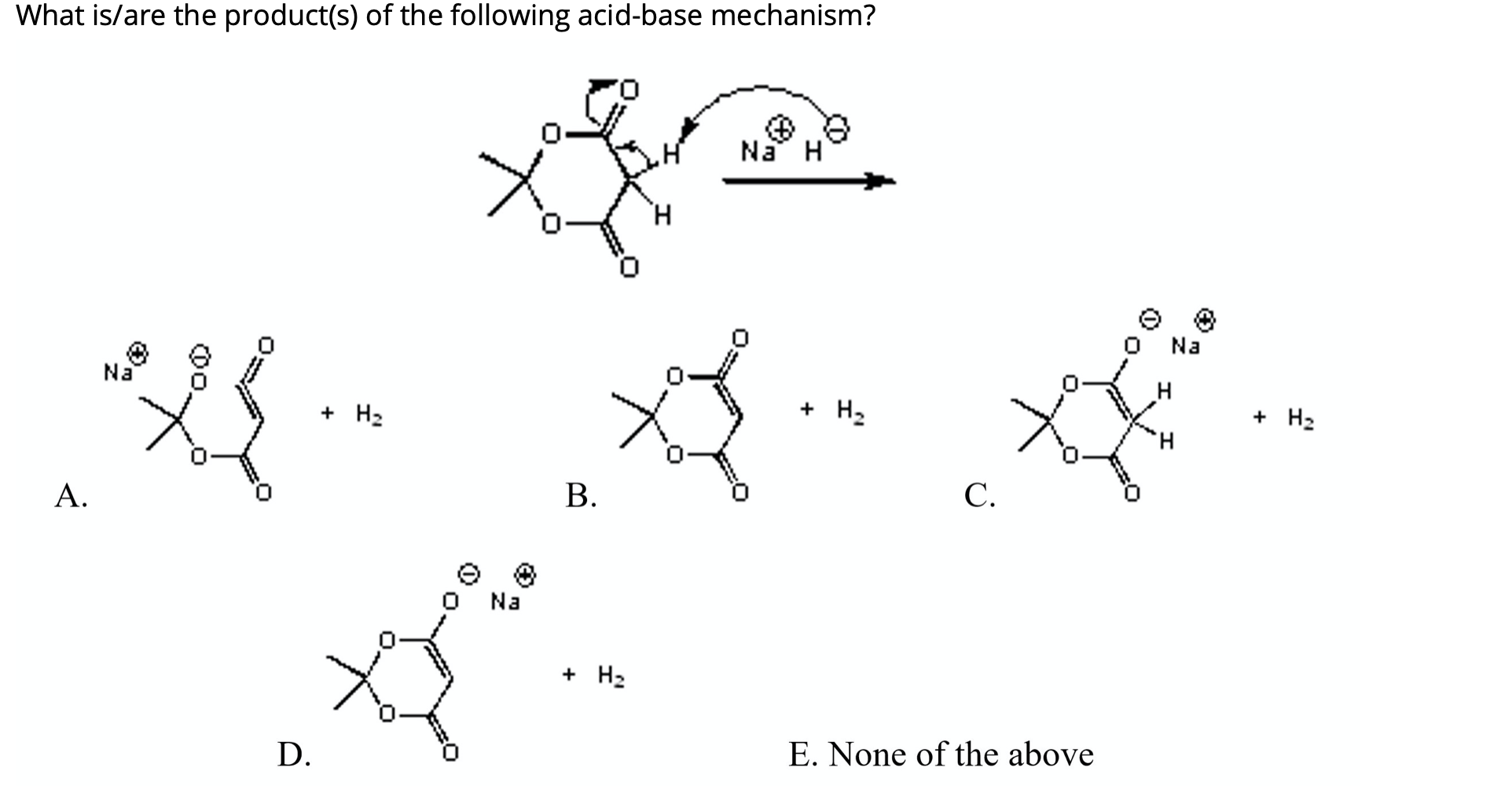
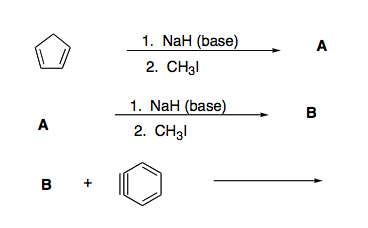
/chapter8/pages9and10/page9and10_files/E2_Zaitsev.png)